Osmosis measures the natural tendency for two aqueous solutions that differ in concentration and are separated by a semi-permeable membrane to approach equal concentrations. In the figure below, what will happen next?
Note that the solution inside the tube is more concentrated than the solution inside the beaker . 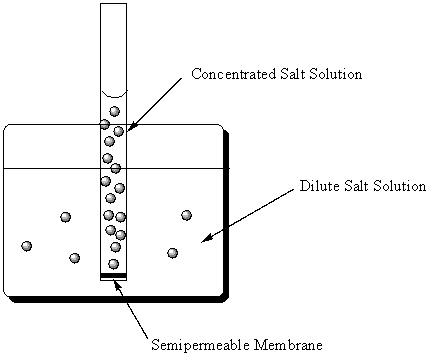
A) Salt will flow from the beaker into the tube and the solution level inside the tube will rise.
B) Water will flow from the beaker into the tube and salt will flow from the tube into the beaker and the solution level inside the tube will remain unchanged.
C) Water will flow from the beaker into the tube and the solution level inside the tube will rise.
D) Water will flow from the tube into the beaker and the solution level inside the tube will lower.
E) Salt will flow from the tube into the beaker and the solution level inside the tube will lower.
Correct Answer:
Verified
Q150: What is the freezing point of a
Q151: A quantity of 7.85 g of a
Q152: A 0.500 gram sample was dissolved in
Q153: What is the freezing point of a
Q154: What is the boiling point of a
Q156: What mass of glycerol (C3H8O3) must be
Q157: What is the freezing point for a
Q158: What is the boiling point of an
Q159: Lauryl alcohol is obtained from coconut oil
Q160: Water has a kf of 1.86 °
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents