Exhibit 13-3 Consider the following reaction and the corresponding time-concentration table to answer the following question(s) . C2H4 (g) + O3 (g) →C2H4O (g) + O2 (g) The concentration of ozone, O3, was monitored for this reaction as a function of time and is given in the table that follows 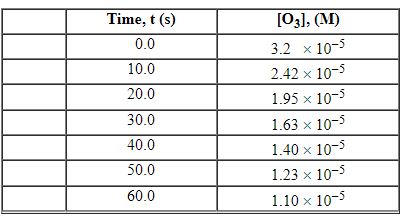
-Refer to Exhibit 13-3. What is the average rate for the time interval from 20.0 seconds tO60.0 seconds?
A) 2.13×10 - 7 M/s
B) 3.5×10 - 7 M/s
C) 3.66×10 - 7 M/s
D) 1.83×10 - 6 M/s
E) 1.40×10 - 5 M/s
Correct Answer:
Verified
Q1: A sample of 0.200 moles of NO
Q2: Exhibit 13-2 Use the data set below
Q3: Exhibit 13-3 Consider the following reaction and
Q5: Exhibit 13-2 Use the data set below
Q6: Which of the following would increase the
Q8: Exhibit 13-2 Use the data set below
Q9: Consider the reaction in aqueous solution:
3 I
Q10: Exhibit 13-2 Use the data set below
Q11: The diagram below is a time-concentration curve
Q17: Exhibit 13-3 Consider the following reaction and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents