Exhibit 13-11 Consider the data that were collected for the rate of disappearance of NO in the reaction below to answer the following question(s) . 2 NO (g) + 2 H2 (g) →N2 (g) + 2 H2O (g) 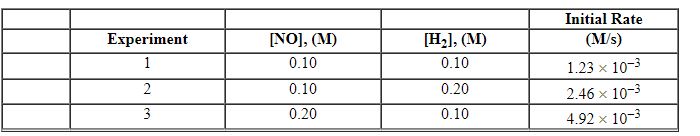
-Refer to Exhibit 13-11. What is the value and units of the rate constant, k , for this reaction?
A) 0.062 M - 1s - 1
B) 0.62 M - 2s - 1
C) 6.2 M - 3s - 1
D) 1.2 M - 2sec - 1
E) 12 M - 3s - 1
Correct Answer:
Verified
Q69: Exhibit 13-9 Use the reaction below to
Q70: Exhibit 13-12 Consider the aqueous reaction and
Q71: Exhibit 13-8 Consider the gas phase reaction
Q72: Exhibit 13-10 Consider the gas phase reaction
Q73: Exhibit 13-12 Consider the aqueous reaction and
Q75: Exhibit 13-10 Consider the gas phase reaction
Q76: Exhibit 13-12 Consider the aqueous reaction and
Q77: For a reaction with the rate law
Q78: Exhibit 13-12 Consider the aqueous reaction and
Q79: Consider the Time-Concentration plots for each of
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents