Exhibit 13-12 Consider the aqueous reaction and data below to answer the following question(s) . 2 HgCl2 (aq) + C2O42 - (aq) →2 Cl - (aq) + 2 CO2 (g) + HgCl2 (s) 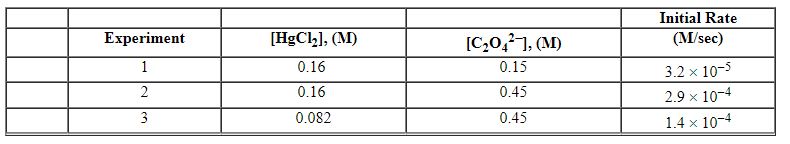
-Refer to Exhibit 13-12. What is the overall order of the rate law?
A) zero order
B) first order
C) second order
D) third order
E) fourth order
Correct Answer:
Verified
Q73: Exhibit 13-12 Consider the aqueous reaction and
Q74: Exhibit 13-11 Consider the data that were
Q75: Exhibit 13-10 Consider the gas phase reaction
Q76: Exhibit 13-12 Consider the aqueous reaction and
Q77: For a reaction with the rate law
Q79: Consider the Time-Concentration plots for each of
Q80: Exhibit 13-13 Graphical analysis can be used
Q81: The half-life of the first order radioactive
Q82: Tritium is radioactive and decays by a
Q83: The reaction A + 2 B→Products was
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents