The half-life of the first order radioactive decay of 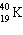 is 1.30 10 9 years. How long would it take for the
is 1.30 10 9 years. How long would it take for the 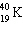 to decay to 25% of its original concentration?
to decay to 25% of its original concentration?
A) 5.4×109 yr
B) 1.30×109 yr
C) 9.75×108 yr
D) 2.60×109 yr
E) 3.25×108 yr
Correct Answer:
Verified
Q76: Exhibit 13-12 Consider the aqueous reaction and
Q77: For a reaction with the rate law
Q78: Exhibit 13-12 Consider the aqueous reaction and
Q79: Consider the Time-Concentration plots for each of
Q80: Exhibit 13-13 Graphical analysis can be used
Q82: Tritium is radioactive and decays by a
Q83: The reaction A + 2 B→Products was
Q84: The half-life of a zero order reaction
Q85: For a reaction that has the rate
Q86: The reaction A + B→C + D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents