The gas phase reaction X + Z Q is found to have the rate law Rate = 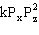 . What will be the effect of doubling the initial pressure of both X and Z on the initial rate?
. What will be the effect of doubling the initial pressure of both X and Z on the initial rate?
A) No effect.
B) Rate goes up by a factor oF4.
C) Rate goes up by a factor of 2.
D) Rate goes up by a factor of 8.
E) Rate goes up by a factor of 16.
Correct Answer:
Verified
Q85: For a reaction that has the rate
Q86: The reaction A + B→C + D
Q87: The decomposition of hydrogen peroxide to water
Q88: The y-intercept on a second order Time-Concentration
Q89: The reaction X + Y→Z has the
Q91: The reaction A + B→C obeys the
Q92: A sample of the radioactive isotope 90Sr
Q93: Exhibit 13-13 Graphical analysis can be used
Q94: The highly toxic pesticide parathion, (C2H5O)2PSOC6H4NO2, decomposes
Q95: For the zero order rate law
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents