The Pepsin-Catalyzed Hydrolysis of Carbobenzene-1-Glutamyl-1-Tyrosine Ethyl Ester Was Found to Have
The pepsin-catalyzed hydrolysis of carbobenzene-1-glutamyl-1-tyrosine ethyl ester was found to have the following relation between rate constant and temperature:
K = 2.66×108 mol/L × s at 24 ° C and k = 14.4×108 mol/L × s at 38 ° C. Given (R = 8.314 J/mol × K) , the activation energy for this reaction in J × mol - 1 can be calculated using:
A) E A = 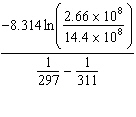
B) E A = 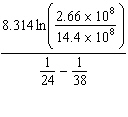
C) E A = 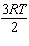
D) E A = 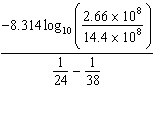
E) E A = 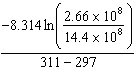
Correct Answer:
Verified
Q135: Compare the following two potential energy diagrams
Q136: Calculate the energy of activation of a
Q137: Arrhenius proposed the equation shown below that
Q138: The decomposition of hydrogen iodide is shown
Q139: Exhibit 13-17 Consider the diagrams shown below
Q141: The reaction X + 2 Y A
Q142: The reaction H3C - I + OH
Q143: Reactions I through III represent the elementary
Q144: The mechanism for NO2 (g) + CO
Q145: Exhibit 13-18 Consider the two-step mechanism below
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents