Below in the figure of a Reaction Energy Diagram is labeled two parameters, x and y . What are these two parameters and is this reaction endothermic or exothermic ?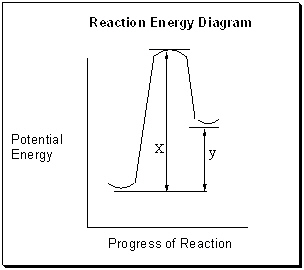
A) "x" is the energy of activation, "y" is the heat of the reaction and this reaction is endothermic.
B) "x" is the energy of activation, "y" is the heat of the reaction and this reaction is exothermic.
C) "x" is the heat of the reaction, "y" is the energy of activation and this reaction is endothermic.
D) "x" is the heat of the reaction, "y" is the energy of activation and this reaction is exothermic.
E) "x" is the heat of the transition state, "y" is the heat of the reaction and this reaction is exothermic.
Correct Answer:
Verified
Q116: Consider the following second order reaction with
Q117: The half-life of the first order radioactive
Q118: A reaction in solution, A + B→P,
Q119: Exhibit 13-14 Consider the decomposition of N2O5
Q120: A biochemist studying the breakdown in soil
Q122: Which of the following will decrease the
Q123: The rate constant for the first order
Q124: At a minimum, what experimental data is
Q125: Why does temperature affect the rate of
Q126: A catalyst:
A) increases the rate of reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents