What can be stated about the two potential energy diagrams shown below with respect to their relative rates of reaction and heat exchange?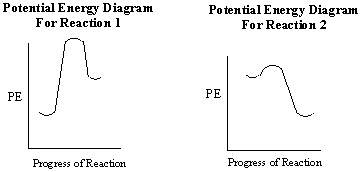
A) Reaction 1 proceeds faster than reaction 2. Reaction 1 is exothermic and reaction 2 is endothermic .
B) Reaction 1 proceeds faster than reaction 2. Reaction 1 is endothermic and reaction 2 is exothermic .
C) Reaction 1 proceeds faster than reaction 2. Both reactions are exothermic .
D) Reaction 2 proceeds faster than reaction 1. Reaction 1 is exothermic and reaction 2 is endothermic .
E) Reaction 2 proceeds faster than reaction 1. Reaction 1 is endothermic and reaction 2 is exothermic .
Correct Answer:
Verified
Q127: A catalyst may be defined as:
A) a
Q128: In an Arrhenius plot where the ln(
Q129: Exhibit 13-17 Consider the diagrams shown below
Q130: The rate constant of a certain reaction
Q131: A certain first-order reaction has a rate
Q133: Which of the following quantities is least
Q134: What is the energy of activation, Ea,
Q135: Compare the following two potential energy diagrams
Q136: Calculate the energy of activation of a
Q137: Arrhenius proposed the equation shown below that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents