At 700 C, the equilibrium constant ( K c ) for the reaction
N₂(g) + 3 H₂ (g) 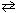 2 NH₃(g) is 2.37 10 - 3 .
2 NH₃(g) is 2.37 10 - 3 .
If 0.683 mol of N₂, 8.80 mol of H₂ and 0.744 mole of NH₃are mixed in a 1.00 L container at 700 C:
A) the value of Q is unknown.
B) the value of Q is greater than K c.
C) the value of Q is equal to K c.
D) the reaction is at equilibrium.
E) the value of Q is less than K c.
Correct Answer:
Verified
Q48: For the reaction
2 NO (g) + 2
Q49: At 1000 K, the value of the
Q50: Under what conditions does changing the pressure
Q51: The reaction NO (g) + O3(g)
Q52: At 100 C, the equilibrium constant for
Q54: The reaction of chlorine and water vapor
Q55: For the reaction 2 NH₃(g) 
Q56: The reaction 2 NO (g) + 2
Q57: Which of the following reactions would shift
Q58: Consider the following reaction:
2 HBr (g)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents