Which of the following reactions would shift to the right to favor the product side upon increasing the pressure (through a reduction in the volume of the reaction vessel) ?
I. H₂ (g) + C₂ N₂(g) 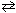 2 HCN (g)
2 HCN (g)
II. CO (g) + Br₂(g) 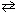 CO Br₂(g)
CO Br₂(g)
III. 6 CO₂ (g) + 6 H₂ O ( 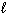 )
) 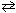 C6 H12 O6 (s) + 6 O₂(g)
C6 H12 O6 (s) + 6 O₂(g)
A) I only
B) II only
C) III only
D) I and III
E) II and III
Correct Answer:
Verified
Q52: At 100 C, the equilibrium constant for
Q53: At 700 C, the equilibrium constant (
Q54: The reaction of chlorine and water vapor
Q55: For the reaction 2 NH₃(g) 
Q56: The reaction 2 NO (g) + 2
Q58: Consider the following reaction:
2 HBr (g)
Q59: For the reaction CO₂ (g) + H₂
Q60: Consider the Haber process for preparing ammonia
Q61: At 125 C, K p = 5.2*10-
Q62: Which of the following reactions is product
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents