Consider the Haber process for preparing ammonia from nitrogen and hydrogen as shown below and the reaction s corresponding equilibrium constant at 532 C.
N₂(g) + 3 H₂ (g) 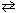 2 NH₃(g) K eq (532 C) = 0.19
2 NH₃(g) K eq (532 C) = 0.19
At a particular moment, the following equilibrium concentrations were observed:
N₂= 0.079 M, H₂ = 0.12 M and NH₃= 0.0051 M. After calculating the reaction quotient , Q , what can be stated about the direction in which this reaction is proceeding?
A) Not enough information is provided to make an assessment.
B) This reaction is proceeding left to right as written. (reactants to products)
C) This reaction is proceeding right to left as written. (product to reactants)
D) This reaction is at a state of chemical equilibrium.
Correct Answer:
Verified
Q55: For the reaction 2 NH₃(g) 
Q56: The reaction 2 NO (g) + 2
Q57: Which of the following reactions would shift
Q58: Consider the following reaction:
2 HBr (g)
Q59: For the reaction CO₂ (g) + H₂
Q61: At 125 C, K p = 5.2*10-
Q62: Which of the following reactions is product
Q63: Which system at equilibrium shifts right ?
I.
Q64: For the high temperature equilibrium system, 2
Q65: Which system at equilibrium shifts right upon
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents