At equilibrium, the system N₂O4 (g) 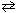 2 NO₂ (g)
2 NO₂ (g)
Was found to have PNO 2= 0.86 atm and P N2O = 0.12 atm. Another equilibrium system of
N₂O4 (g) 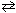 2 NO₂ (g)
2 NO₂ (g)
(at the same temperature as the first) was found to have P NO = 0.98 atm. The pressure of N₂O4 in the second system was:
A) 0.11 atm
B) 0.17 atm
C) 0.13 atm
D) 0.16 atm
E) none of these
Correct Answer:
Verified
Q110: At 1400 K, the value of K
Q111: For the reaction COCl₂ (g) 
Q112: The equilibrium constant ( K p )
Q113: The equilibrium constant K c is 10
Q114: Consider the following system at equilibrium. A
Q116: The equilibrium constant K c is 3.00
Q117: Consider the following reaction. Its equilibrium constant
Q118: Exhibit 14-3 Consider the reaction below and
Q119: K p for COCl₂ (g) 
Q120: At 525 C, K p = 0.250
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents