At 525 C, K p = 0.250 for CO₂ (g) + H₂ (g) 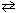 CO (g) + H₂ O (g) . If enough CO₂ and H₂ is injected into a container so that CO₂ and H₂ each exert a partial pressure of 1.000 atm (P total = 2.000 atm) , what is the final partial pressure of H₂ at equilibrium at 525 C?
CO (g) + H₂ O (g) . If enough CO₂ and H₂ is injected into a container so that CO₂ and H₂ each exert a partial pressure of 1.000 atm (P total = 2.000 atm) , what is the final partial pressure of H₂ at equilibrium at 525 C?
A) 0.333 atm
B) 0.500 atm
C) 0.667 atm
D) 0.250 atm
E) 2.000 atm
Correct Answer:
Verified
Q115: At equilibrium, the system N₂O4 (g)
Q116: The equilibrium constant K c is 3.00
Q117: Consider the following reaction. Its equilibrium constant
Q118: Exhibit 14-3 Consider the reaction below and
Q119: K p for COCl₂ (g) 
Q121: Exhibit 14-5 Consider the decomposition reaction of
Q122: What is the equilibrium constant expression for
Q123: What is the equilibrium constant expression for
Q124: What would be the equilibrium constant expression
Q125: At 1073 K, the pressure of CO₂
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents