Exhibit 14-5 Consider the decomposition reaction of Hydrogen sulfide, H₂ S, and the corresponding equilibrium constant as shown below to answer the following question(s) :
2 H₂ S (g) 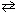 2 H₂ (g) + S2(g) K c = 9.30*10- 8
2 H₂ (g) + S2(g) K c = 9.30*10- 8
Complete the following ICE table for when 0.150 moles of H₂ S is placed in a 1.00 liter container. Molar Conc, M H₂ S H₂ S2Initial Change At equilibrium
-Refer to Exhibit 14-5. What equilibrium expression shown below would allow you to solve for x and therefore enable you to solve for the equilibrium concentrations of each of the substances present at equilibrium?
A) x3/(0.15 - x) 2 = 9.30×10 - 8
B) 4x3/(0.15 - 2x) 2 = 9.30×10 - 8
C) 2x3/(0.15 - 2x) 2 = 9.30×10 - 8
D) 4x3/(0.15 - x) 2 = 9.30×10 - 8
E) 2x3/(0.15 + 2x) 2 = 9.30×10 - 8
Correct Answer:
Verified
Q116: The equilibrium constant K c is 3.00
Q117: Consider the following reaction. Its equilibrium constant
Q118: Exhibit 14-3 Consider the reaction below and
Q119: K p for COCl₂ (g) 
Q120: At 525 C, K p = 0.250
Q122: What is the equilibrium constant expression for
Q123: What is the equilibrium constant expression for
Q124: What would be the equilibrium constant expression
Q125: At 1073 K, the pressure of CO₂
Q126: For the reaction NH ₄NO₂ (s)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents