Exhibit 14-3 Consider the reaction below and its corresponding equilibrium constant at 2000 C to answer the following question(s) :
N₂(g) + O₂(g) 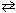 2 NO (g) K c = 4.10*10- 4 (at 2000 C) Complete the table shown below for an experiment where 0.55 moles of N₂is mixed with 0.45 moles of O₂in a 1.00 Liter reaction vessel and the reaction mixture is allowed to come to equilibrium. Concentration N₂O₂ 2 NO Initial 0.55 M 0.45 M Change At equilibrium
2 NO (g) K c = 4.10*10- 4 (at 2000 C) Complete the table shown below for an experiment where 0.55 moles of N₂is mixed with 0.45 moles of O₂in a 1.00 Liter reaction vessel and the reaction mixture is allowed to come to equilibrium. Concentration N₂O₂ 2 NO Initial 0.55 M 0.45 M Change At equilibrium
-Refer to Exhibit 14-3. What equilibrium expression shown below would allow you to solve for x and therefore enable you to solve for the equilibrium concentrations of each of the substances present at equilibrium?
A) x2/(0.55 - x) (0.45 - x) = 4.1×10 - 4
B) 2x2/(0.55 + x) (0.45 + x) = 4.1×10 - 4
C) 2x2/(0.55 - x) (0.45 - x) = 4.1×10 - 4
D) 4x2/(0.55 - x) (0.45 - x) = 4.1×10 - 4
E) 2x2/(0.55 + x) (0.450 + x) = 4.1×10 - 4
Correct Answer:
Verified
Q103: For the reaction H₂ (g) + CO₂
Q104: Consider the following reaction:
H₂ (g) + I2
Q105: At 457 K, the value of K
Q106: Given that K eq = 2.4*103 for
Q107: K c = 0.35 for the reaction:
H₂
Q109: At 1400 K, the reaction 2 HBr
Q110: At 1400 K, the value of K
Q111: For the reaction COCl₂ (g) 
Q112: The equilibrium constant ( K p )
Q113: The equilibrium constant K c is 10
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents