For the reaction H₂ (g) + CO₂ (g) 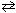 H₂ O (g) + CO (g) at 700 C, K c = 0.534. Calculate the concentration of H₂ at equilibrium if a mixture of 0.300 mol of H₂ O, 0.300 mol CO, 0.100 mol H₂ and 0.100 mol CO₂ is heated to 700 C in a 1.00 liter container.
H₂ O (g) + CO (g) at 700 C, K c = 0.534. Calculate the concentration of H₂ at equilibrium if a mixture of 0.300 mol of H₂ O, 0.300 mol CO, 0.100 mol H₂ and 0.100 mol CO₂ is heated to 700 C in a 1.00 liter container.
A) 0.300 M
B) 0.100 M
C) 0.131 M
D) 0.231 M
E) 0.169 M
Correct Answer:
Verified
Q98: At 500 C the equilibrium concentrations are
Q99: Which of the items listed below would
Q100: What is the equilibrium constant, K p
Q101: Exhibit 14-4 Consider the reaction below and
Q102: K p at a certain temperature for
Q104: Consider the following reaction:
H₂ (g) + I2
Q105: At 457 K, the value of K
Q106: Given that K eq = 2.4*103 for
Q107: K c = 0.35 for the reaction:
H₂
Q108: Exhibit 14-3 Consider the reaction below and
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents