At 500 C the equilibrium concentrations are NH₃= 0.0596 M , N₂= 0.600 M , and H₂ = 0.420 M for the reaction N₂(g) + 3 H₂ (g) 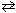 2 NH₃(g) . Calculate K c .
2 NH₃(g) . Calculate K c .
A) 0.237
B) 4.23
C) 0.0799
D) 12.5
E) 40.0
Correct Answer:
Verified
Q93: A quantity of PCl5 gas is heated.
Q94: For the Haber reaction at equilibrium ,
Q95: When the reversible reaction, N₂+ O₂
Q96: An equilibrium mixture at 500 K has
Q97: A reaction vessel is charged with 1.720
Q99: Which of the items listed below would
Q100: What is the equilibrium constant, K p
Q101: Exhibit 14-4 Consider the reaction below and
Q102: K p at a certain temperature for
Q103: For the reaction H₂ (g) + CO₂
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents