A quantity of PCl5 gas is heated. At equilibrium the molar concentrations of PCl5 , PCl 3 , and Cl₂ gases were 0.008 M , 0.02 M , and 0.02 M , respectively. What is the value of K c for:
PCl5 (g) 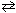 PCl 3 (g) + Cl₂ (g)
PCl 3 (g) + Cl₂ (g)
A) 0.5
B) 20.0
C) 0.05
D) 20
E) none of these
Correct Answer:
Verified
Q88: At 25 C, K p = 0.240
Q89: Consider the following reaction at equilibrium:
2 Cl₂
Q90: Which of the items listed below would
Q91: Sulfur trioxide decomposes at high temperature in
Q92: Consider the reaction:
2 HI (g) 
Q94: For the Haber reaction at equilibrium ,
Q95: When the reversible reaction, N₂+ O₂
Q96: An equilibrium mixture at 500 K has
Q97: A reaction vessel is charged with 1.720
Q98: At 500 C the equilibrium concentrations are
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents