The K c for the reaction,
PCl5 (g) 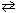 PCl3(g) + Cl₂ (g) , at a particular temperature is 4.0*10- 2 .
PCl3(g) + Cl₂ (g) , at a particular temperature is 4.0*10- 2 .
How many moles of PCl5 must be placed in an empty 1.0 liter flask to have the equilibrium concentration of Cl₂ = 0.15 M ?
A) 0.71
B) 0.15
C) 1.4
D) 6.7
E) 0.23
Correct Answer:
Verified
Q130: Exhibit 14-5 Consider the decomposition reaction of
Q131: Exhibit 14-5 Consider the decomposition reaction of
Q132: What is the equilibrium constant expression for
Q133: Exhibit 14-4 Consider the reaction below and
Q134: A simplifying assumption can be made when
Q136: For the reaction
NH ₄NO₂ (s) 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents