The reaction 2 NaHSO₄(s) 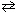 Na2 S2O 7 (s) + H₂ O (g) is endothermic at 298 K. Therefore,
Na2 S2O 7 (s) + H₂ O (g) is endothermic at 298 K. Therefore,
A) D H ° rxn is positive.
B) The partial pressure of H2O would be higher at 500K.
C) K p would increase with increasing temperature.
D) All of a, b, and c are correct.
E) None of a, b, and c are correct.
Correct Answer:
Verified
Q132: What is the equilibrium constant expression for
Q133: Exhibit 14-4 Consider the reaction below and
Q134: A simplifying assumption can be made when
Q135: The K c for the reaction,
PCl5 (g)
Q136: For the reaction
NH ₄NO₂ (s) 
Q138: What is the concentration equilibrium constant expression
Q139: What is the equilibrium constant expression for
Q140: For the reaction Fe (s) + 4
Q141: Which of the following sulfide salts is
Q142: Which of the following iodates would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents