The endothermic reaction NH ₄HS (s) 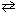 NH₃(g) + H₂ S (g)
NH₃(g) + H₂ S (g)
Takes place in a container at high temperature and high pressure. Therefore:
A) increased temperature favors NH4HS.
B) increased temperature favors NH3.
C) a maximum amount of NH3 is formed.
D) the results may not be predicted because high T and high P are opposing effects.
E) none of these statements is correct.
Correct Answer:
Verified
Q143: In water, the solubility of Al(OH)3 is
Q144: The solubility of CaF2 in water is
Q145: The solubility of Tl2CrO4 in pure water
Q146: The equilibrium constant K p for the
Q147: For the reaction, Ag 3 PO4 (s)
Q149: The solubility of BaSO4 is 1.14×10 -
Q150: The solubility of Pb(IO3)2 in water is
Q151: At a particular temperature, the solubility of
Q152: At a particular temperature, the hydroxide ion
Q153: Which of the following sulfates is least
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents