Consider solving for the molar solubility of a 3:1 electrolyte such as Ag3PO4 . Ag3PO4 (s) 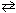 3 Ag 1+ (aq) + PO4 3 - (aq)
3 Ag 1+ (aq) + PO4 3 - (aq)
After constructing an ICE table and writing a solubility constant expression for this process, what solubility product expression listed below would be suitable for solving for x which could then be related to the equilibrium concentrations of Ag 1+ and PO4 3 - ion concentrations and through stoichiometry can be related to the solubility of Ag 3 PO4 in water?
A) K sp = 3x3
B) K sp = 27x3
C) K sp = x4
D) K sp = 3x4
E) K sp = 27x4
Correct Answer:
Verified
Q165: The solubility product constant for Ca3(PO4)2 is
Q166: The K sp for silver bromide (AgBr)
Q167: What is the solubility of strontium fluoride,
Q168: If the molar solubility of Silver Phosphate,
Q169: What is the concentration of Ag+ ions
Q171: What is the molar solubility of AgIO3
Q172: What is the concentration of Ag+ ions
Q173: The K sp for PbBr2 is 4.0×10
Q174: Consider solving for the molar solubility of
Q175: The K sp of Mg3(PO4)2 is 1.0×10
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents