Exhibit 14-7 Consider dissolving LaF 3 in water as shown below to answer the following question(s) :
LaF3 (s) 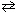 La 3+ (aq) + 3 F - (aq) K sp = 2.0*10- 19
La 3+ (aq) + 3 F - (aq) K sp = 2.0*10- 19
-Refer to Exhibit 14-7. The solubility product constant for LaF3 is 2.0*10- 19 . Which expression listed below would be used to solve for the molar solubility of LaF 3 in water?
A) 2.0×10 - 19 = x4
B) 2.0×10 - 19 = 3x2
C) 2.0×10 - 19 = 3x4
D) 2.0×10 - 19 = 4x4
E) 2.0×10 - 19 = 27x4
Correct Answer:
Verified
Q188: Calculate the solubility of AgI in 0.10
Q189: What is the minimum concentration of Br
Q190: Mg(OH)2 ( K sp = 8.9×10 -
Q191: What is the molar solubility of PbBr2
Q192: What is the molar solubility of Ag2SO4
Q194: Calculate the ion product value, Q sp,
Q195: What is the solubility of PbSO4 (
Q196: What is the molar solubility of PbI2
Q197: A mixture of NaCl, NaF, KOH, and
Q198: What is the concentration of Pb 2+
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents