What is the concentration of Pb 2+ ions in a solution resulting from the addition of 1.0 10 - 3 moles of Na2 CrO4 to 1.0 liter of solution containing solid PbCrO4 ( K sp = 1.8*10- 14 ) in equilibrium with its ions?
(Assume no volume change on addition of Na2 CrO4 .) PbCrO4 (s) 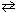 Pb 2+ (aq) + CrO₂ - (aq)
Pb 2+ (aq) + CrO₂ - (aq)
A) 1.8×10 - 17 M
B) 1.34×10 - 7 M
C) 1.0×10 - 3 M
D) 1.34×10 - 10 M
E) 1.8×10 - 11 M
Correct Answer:
Verified
Q193: Exhibit 14-7 Consider dissolving LaF 3 in
Q194: Calculate the ion product value, Q sp,
Q195: What is the solubility of PbSO4 (
Q196: What is the molar solubility of PbI2
Q197: A mixture of NaCl, NaF, KOH, and
Q199: Exhibit 14-7 Consider dissolving LaF 3 in
Q200: A mixture of Na2CO3, NaCl, and NiCO3
Q201: Lead(II) chloride has limited solubility in water
Q202: Which ionic compound(s) listed below would increase
Q203: A solution is prepared such that the
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents