Aniline, C6 H5 NH₂ , is commonly used as a precursor for making dyes. Aniline is a base when dissolved in water. It ionizes as shown below. What is the base ionization constant, K b , of aniline if an aqueous 0.20 M C6 H5 NH₂ solution has a pOH value equal tO5 .05?
C6 H5 NH₂ + H₂ O 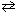 C6 H5 NH₃+ + OH -
C6 H5 NH₃+ + OH -
A) K b = 6.3×10 - 18
B) K b = 4.0×10 - 10
C) K b = 4.5×10 - 5
D) K b = 1.5×10 - 2
E) K b = 6.3×1010
Correct Answer:
Verified
Q99: The hydrogen ion concentration of a 0.0010
Q100: A 0.20 M solution of the weak
Q101: Which statement below is true about an
Q102: Which of the following ammonium salts would
Q103: The pH of a 1.00 liter solution
Q105: Which salt(s) listed below form(s) an acidic
Q106: What is the pH of a 0.20
Q107: Consider the salt ammonium fluoride, NH4F, that
Q108: Which of the following salts is(are) considered
Q109: Exhibit 15-3 Ephedrine (C10H15ON), a central nervous
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents