Exhibit 15-3 Ephedrine (C10H15ON) , a central nervous system stimulant, is used in nasal sprays as a decongestant. This compound is a weak organic base :
C10H15ON (aq) + H₂ O 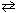 HC10H15ON + (aq) + OH - (aq) Consider a 0.035 M
HC10H15ON + (aq) + OH - (aq) Consider a 0.035 M
Solution of ephedrine that has a pH of 11.33 to answer the following problem(s) .
-Refer to Exhibit 15-3. What is the equilibrium concentration of ephedrine, C10H15ON eq , in this 0.035 M solution?
A) 4.7×10 - 12 M
B) 2.1×10 - 3 M
C) 3.3×10 - 2 M
D) 3.7×10 - 2 M
E) 4.5×10 - 2 M
Correct Answer:
Verified
Q104: Aniline, C6 H5 NH₂ , is commonly
Q105: Which salt(s) listed below form(s) an acidic
Q106: What is the pH of a 0.20
Q107: Consider the salt ammonium fluoride, NH4F, that
Q108: Which of the following salts is(are) considered
Q110: Which of the following salts is considered
Q111: Which salt(s) listed below form(s) an acidic
Q112: Which of the following salts is(are) considered
Q113: Which of the following salts is(are) considered
Q114: Which of the following salts would be
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents