Consider titrating a triprotic acid with standard NaOH as shown in the titration curve below. 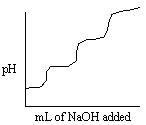 What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
What is the relationship between the first equivalence point and the third equivalence point in this titration curve?
A) The pH of the first equivalence point is three times larger than the pH found at the third equivalence point.
B) The pH of the first equivalence point is three times smaller than the pH found at the third equivalence point.
C) The volume of titrant required to reach the third equivalence point is three times as large as the volume of titrant required to reach the first equivalence point.
D) The volume of titrant required to reach the first equivalence point is three times as large as the volume of titrant required to reach the third equivalence point.
E) There are no relationships between the first and third equivalence points.
Correct Answer:
Verified
Q61: The titration of a weak acid, HA
Q62: Which of the following titration curves listed
Q63: The pH at the equivalence point in
Q64: Exhibit 16-3 Consider the Titration curve below
Q65: Which of the following titrations would produce
Q67: The titration of a weak acid, HA
Q68: In the titration of 25.0 mL of
Q69: Consider the titration of a weak acid
Q70: A 20.0 mL sample of lactic acid
Q71: Which of the following titration curves listed
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents