Which of the following processes results in a negative entropy change?
A) HCN(g, V = 10 L, P = 4 atm) 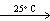 HCN (g, V = 20 L, P = 2 atm)
HCN (g, V = 20 L, P = 2 atm)
B) 2 HI (g) 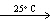 H₂ (g) + I2 (s)
H₂ (g) + I2 (s)
C) NaI (s) 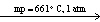 NaI (
NaI ( 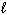 )
)
D) CaCO3(s) 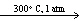 CaO (s) + CO₂ (g)
CaO (s) + CO₂ (g)
E) Bi ( 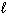 ) + Sn (
) + Sn ( 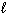 ) + Pb (
) + Pb ( 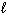 )
) 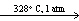 Soft Solder (
Soft Solder ( 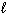 )
)
Correct Answer:
Verified
Q34: Which of the following reactions would be
Q35: Which of the following chemical reactions has
Q36: Which of the following processes would be
Q37: The entropy change when one mole of
Q38: Which of the following reactions would be
Q40: Estimate the normal boiling point of mercury,
Q41: Which of the following processes is(are) considered
Q42: The reaction PCl3 (g) + Cl2 (g)→PCl5
Q43: Which thermodynamic statement listed below would confirm
Q44: What is the criterion for a reaction
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents