Which of the following reactions would be expected to have a positive entropy change, D S 0?
I. 2 SO₂ (g) + O₂(g) 2 SO3(g)
II. Ba(OH) 2 (s) BaO (s) + H₂ O (g)
III. CO (g) + 2 H₂ (g) CH₃ OH ( 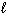 )
)
A) I only
B) II only
C) III only
D) I and II
E) I and III
Correct Answer:
Verified
Q33: Which of the following chemical reactions would
Q34: Which of the following reactions would be
Q35: Which of the following chemical reactions has
Q36: Which of the following processes would be
Q37: The entropy change when one mole of
Q39: Which of the following processes results in
Q40: Estimate the normal boiling point of mercury,
Q41: Which of the following processes is(are) considered
Q42: The reaction PCl3 (g) + Cl2 (g)→PCl5
Q43: Which thermodynamic statement listed below would confirm
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents