Given the abbreviated table for selected standard molar entropy values of each substance in the reaction below, what is the standard molar change in entropy, D S rxn , for the following reaction?
3 NO₂ (g) + H₂ O ( 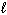 ) 2 HNO3(
) 2 HNO3( 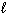 ) + NO (g) Substance S (Jmol - 1 K - 1 ) NO₂ (g) 239.9 H₂ O (
) + NO (g) Substance S (Jmol - 1 K - 1 ) NO₂ (g) 239.9 H₂ O ( 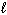 ) 69.94 HNO3(
) 69.94 HNO3( 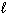 ) 155.6 NO (g) 210.65
) 155.6 NO (g) 210.65
A) D S ° rxn = - 267.8 Jmol - 1K - 1
B) D S ° rxn = - 127.9 Jmol - 1K - 1
C) D S ° rxn = 56.4 Jmol - 1K - 1
D) D S ° rxn = 1171.6 Jmol - 1K - 1
E) D S ° rxn = 1311.5 Jmol - 1K - 1
Correct Answer:
Verified
Q55: For a given chemical reaction, the value
Q56: Which of the following is true?
A) D
Q57: Given D Q58: Which substance(s) listed below would be expected Q59: A positive value of D G ° Q61: If D H is positive and D Q62: Consider the following reaction and its corresponding Q63: Exhibit 17-3 Consider the reaction below and Q64: Consider the following reaction and its corresponding Q65: Exhibit 17-5 Consider the reaction of Barium![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents