Given D 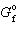 CuO = - 130 kJ/mol and D
CuO = - 130 kJ/mol and D 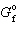 Cu 2 O = - 146 kJ/mol, what is D G for the following reaction?
Cu 2 O = - 146 kJ/mol, what is D G for the following reaction?
4 CuO (s) 2 Cu 2 O (s) + O₂(g)
A) +298 kJ
B) +228 kJ
C) - 7 kJ
D) - 4 kJ
E) not enough information
Correct Answer:
Verified
Q52: Exhibit 17-1 Consider the reaction of carbon
Q53: The best measure of the spontaneity of
Q54: Which of the following processes is(are) considered
Q55: For a given chemical reaction, the value
Q56: Which of the following is true?
A) D
Q58: Which substance(s) listed below would be expected
Q59: A positive value of D G °
Q60: Given the abbreviated table for selected standard
Q61: If D H is positive and D
Q62: Consider the following reaction and its corresponding
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents