Which of the following processes is(are) considered spontaneous ?
I. Melting ice cubes at 5 C and 1 atm.
II. Rust, Fe2 O3 , is converted to iron, Fe, and oxygen, O₂.
III. Forming additional NH₃from the following reaction that has previously reached a state of dynamic equilibrium:
N₂(g) + 3 H₂ (g) 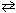 2 NH₃(g)
2 NH₃(g)
A) I only
B) II only
C) III only
D) I and II
E) All of these.
Correct Answer:
Verified
Q49: Given the abbreviated table for selected standard
Q50: Given D Q51: Which substance(s) listed below would be expected Q52: Exhibit 17-1 Consider the reaction of carbon Q53: The best measure of the spontaneity of Q55: For a given chemical reaction, the value Q56: Which of the following is true?![]()
A) D
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents