What is the value of the equilibrium constant, K , at 298 K for the following reaction?
Sn (s) + Pb 2+ (aq) 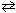 Sn 2+ (aq) + Pb (s) E cell = 0.01 V
Sn 2+ (aq) + Pb (s) E cell = 0.01 V
A) K = 5×10 - 1
B) K = 7×10 - 1
C) K = 8×10 - 1
D) K = 1
E) K = 2
Correct Answer:
Verified
Q94: The products of an electrolysis may depend
Q95: Consider a concentration cell comprised of two
Q96: Given:
2 U3+ + Cd2+→Cd (s) + 2
Q97: Batteries are considered useful sources of electrical
Q98: Consider a voltaic cell that operates using
Q100: What is the cell potential, E cell,
Q101: Electrolysis is NOT generally used to produce:
A)
Q102: A current of 0.452 amperes was passed
Q103: An aqueous solution of potassium nitrate (KNO3)
Q104: How long (in seconds) would it take
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents