Exhibit 22-7 Consider a line-angle drawing of a molecule of Testosterone, a male hormone, to answer the following question(s) . 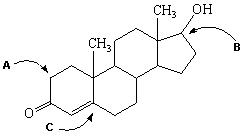 Testosterone
Testosterone
-Refer to Exhibit 22-7. What is the local geometry assumed by each of the labeled carbon atoms in the figure of Testosterone above?
A) A = tetrahedral, B = tetrahedral, C = tetrahedral
B) A = trigonal pyramidal, B = trigonal pyramidal, C = trigonal pyramidal
C) A = trigonal planar, B = trigonal planar, C = trigonal planar
D) A = tetrahedral, B = tetrahedral, C = trigonal planar
E) A = linear, B = trigonal planar, C = trigonal planar
Correct Answer:
Verified
Q136: Morphine, an alkaloid drug that is commonly
Q137: Exhibit 22-3 Consider the molecule of cinnamaldehyde,
Q138: Exhibit 22-4 Cortisol is a hormone that
Q139: Vanillin is a naturally occurring flavoring agent
Q140: Exhibit 22-2 Methadone is a narcotic painkiller
Q142: Exhibit 22-5 Lipoxins are potent anti-inflammatory compounds.
Q143: What approximate bond angles are assumed for
Q144: Using VSEPR theory, what approximate bond angles
Q145: Exhibit 22-5 Lipoxins are potent anti-inflammatory compounds.
Q146: Name the compound pictured below. 
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents