Iodine-131 is used in the treatment of thyroid disorders. 1.0 μg of this isotope produces 3.5 mCi. If a patient is given a dose of 165 mCi, what mass of iodine-131 was in the sample?
A) 0.021 μg
B) 47 μg
C) 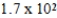 μg
μg
D) 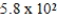 μg
μg
Correct Answer:
Verified
Q22: Which of the following would be classified
Q24: The half-life of 14C is 5,700 years.
Q24: If you absorb 0.0056 rad of energy
Q25: Which of the following reactions represents the
Q26: When uranium-235 fissions the energy is emitted
A)as
Q27: What is the element produced by the
Q28: Which of the following correctly ranks the
Q30: Consider the process illustrated in the image
Q34: Consider the following reaction. Q40: The half-life of is bromine-80 is 17.6![]()
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents