The half-life of 14C is 5,700 years. In a living organism the 14C content is a constant of 0.22 Bq/g. If an unknown sample contains a 14C activity of about 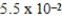 Bq/g , approximately how old is the sample?
Bq/g , approximately how old is the sample?
A) 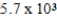 yr
yr
B) 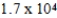 yr
yr
C) 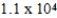 yr
yr
D) 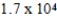 yr
yr
Correct Answer:
Verified
Q2: A scintillation counter is more sensitive in
Q20: The chemical symbol for a positron is:
Q21: Three patients receive equal amounts of energy
Q22: Which of the following would be classified
Q23: One person standing 10 m from a
Q25: Which of the following reactions represents the
Q26: When uranium-235 fissions the energy is emitted
A)as
Q27: What is the element produced by the
Q29: Iodine-131 is used in the treatment of
Q35: Which of the following radiation units takes
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents