Passage
The bacterium Clostridium difficile secretes protein toxins TcdA and TcdB, two homologues associated with C. difficile-related colitis. Tcd proteins enter host intestinal cells via endocytosis induced by interactions between the Tcd C-terminal domain and cell surface receptors. Endocytosis is followed by translocation of the N-terminal glucosyltransferase domain (GTD) across the endosomal membrane. The GTD remains anchored to endosomes by the central region of the protein, a transmembrane domain believed to form membrane pores and facilitate translocation. Inositol hexakisphosphate (IP6) binds Tcd proteins, an interaction that is stabilized by salt bridges in the binding pocket (Figure 1) .
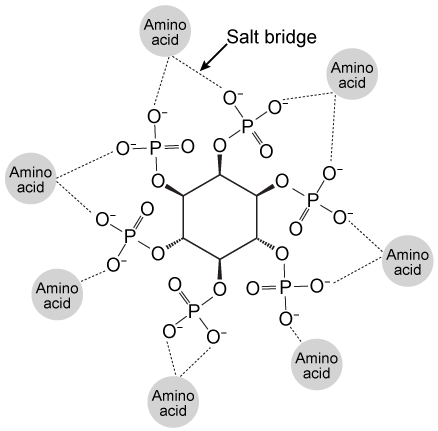 Figure 1 Salt bridges formed between IP6 and the amino acids in the binding pocketThis interaction induces a conformational change that causes a molecular flap to move away from the catalytic site. The catalytic site then mediates intramolecular cleavage in a reaction known as autoprocessing. This reaction results in the release of GTD into the cytosol, where it becomes active.To better understand the relationship between IP6 binding and catalytic activity, autoprocessing assays were performed. Researchers mutated histidine to alanine at positions 759 and 757 in the flap regions of TcdA and TcdB, respectively. They then compared the autoprocessing activities of H759A and H757A mutants to those of wild-type TcdA and TcdB at varying concentrations of IP6 (Figure 2) .
Figure 1 Salt bridges formed between IP6 and the amino acids in the binding pocketThis interaction induces a conformational change that causes a molecular flap to move away from the catalytic site. The catalytic site then mediates intramolecular cleavage in a reaction known as autoprocessing. This reaction results in the release of GTD into the cytosol, where it becomes active.To better understand the relationship between IP6 binding and catalytic activity, autoprocessing assays were performed. Researchers mutated histidine to alanine at positions 759 and 757 in the flap regions of TcdA and TcdB, respectively. They then compared the autoprocessing activities of H759A and H757A mutants to those of wild-type TcdA and TcdB at varying concentrations of IP6 (Figure 2) .
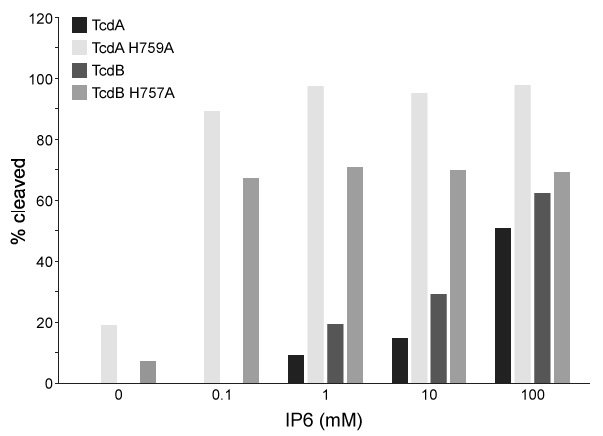 Figure 2 Percent cleavage of wild-type and mutant Tcd by autoprocessing
Figure 2 Percent cleavage of wild-type and mutant Tcd by autoprocessing
Adapted from Chumbler NM, Rutherford SA, Zhang Z, et al. Crystal structure of Clostridium difficile toxin A. Nat Microbiol. 2016;1:15002.
-Based on information given in the passage, stabilizing interactions are most likely to occur between phosphate groups on IP6 and which of the following amino acids lining the Tcd binding pocket?
A) Lys704 via electrostatic interactions
B) Gly705 via hydrophobic interactions
C) Cys707 via covalent bonding
D) Asp709 via ionic interactions
Correct Answer:
Verified
Q21: Passage
The bacterium Clostridium difficile secretes protein toxins
Q22: Passage
Spinocerebellar ataxia 3 (SCA3) is a neurodegenerative
Q23: Passage
Spinocerebellar ataxia 3 (SCA3) is a neurodegenerative
Q24: Passage
The liver plays a central role in
Q25: Passage
The innate immune system relies heavily on
Q27: Passage
The innate immune system relies heavily on
Q28: Passage
The liver plays a central role in
Q29: Passage
The liver plays a central role in
Q30: Passage
The bacterium Clostridium difficile secretes protein toxins
Q31: Passage
Spinocerebellar ataxia 3 (SCA3) is a neurodegenerative
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents