Passage
Dystroglycan (Dg) is a transmembrane protein that mediates interactions between the cytoskeleton and the extracellular matrix (ECM) . It binds the ECM protein laminin in an interaction that is required for intercellular communication and muscle membrane structural stability. The Dg-laminin interaction is facilitated by a long carbohydrate chain called an O-mannosyl glycan, which is covalently linked to Dg (Figure 1) .
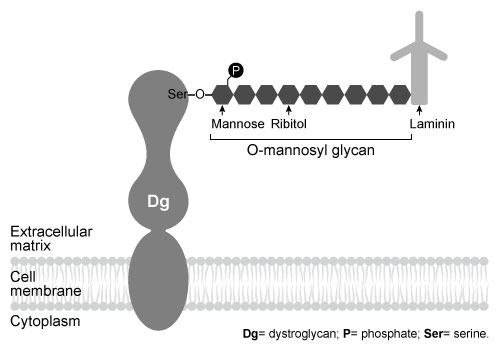 Figure 1 Schematic of interaction between Dg and laminin, mediated by the Dg-linked O-mannosyl glycanThe O-mannosyl glycan is added to Dg by a series of enzymes called glycosyltransferases. The first enzyme in this series, protein O-mannosyltransferase (POMT) , attaches D-mannose to the hydroxyl groups of serine and threonine residues in an α-linkage through the anomeric carbon. The next sugar in the chain then forms a β-1,4 linkage to the mannose residue, followed by several more steps to produce the full glycan. Genetic inactivation of any of the enzymes in the pathway results in an incomplete glycan and failure to bind laminin, and is the underlying cause of several severe forms of muscular dystrophy known as dystroglycanopathies.Researchers investigating dystroglycanopathies recently discovered the functions of two enzymes in the pathway: protein O-mannose kinase (POMK) and fukutin. POMK phosphorylates Dg-linked mannose at carbon 6, and fukutin adds the sugar ribitol 5-phosphate to the glycan chain. To assess the roles of POMK and fukutin relative to each other, scientists conducted experiments in which lysates from muscle cells deficient in either POMK or fukutin were incubated with 32P-labeled ATP or 3H-labeled activated ribitol 5-phosphate. Combined lysates containing both fukutin and POMK were also tested. Dg was then purified and detected by autoradiography and western blot using an antibody against Dg, as shown in Figure 2.
Figure 1 Schematic of interaction between Dg and laminin, mediated by the Dg-linked O-mannosyl glycanThe O-mannosyl glycan is added to Dg by a series of enzymes called glycosyltransferases. The first enzyme in this series, protein O-mannosyltransferase (POMT) , attaches D-mannose to the hydroxyl groups of serine and threonine residues in an α-linkage through the anomeric carbon. The next sugar in the chain then forms a β-1,4 linkage to the mannose residue, followed by several more steps to produce the full glycan. Genetic inactivation of any of the enzymes in the pathway results in an incomplete glycan and failure to bind laminin, and is the underlying cause of several severe forms of muscular dystrophy known as dystroglycanopathies.Researchers investigating dystroglycanopathies recently discovered the functions of two enzymes in the pathway: protein O-mannose kinase (POMK) and fukutin. POMK phosphorylates Dg-linked mannose at carbon 6, and fukutin adds the sugar ribitol 5-phosphate to the glycan chain. To assess the roles of POMK and fukutin relative to each other, scientists conducted experiments in which lysates from muscle cells deficient in either POMK or fukutin were incubated with 32P-labeled ATP or 3H-labeled activated ribitol 5-phosphate. Combined lysates containing both fukutin and POMK were also tested. Dg was then purified and detected by autoradiography and western blot using an antibody against Dg, as shown in Figure 2.
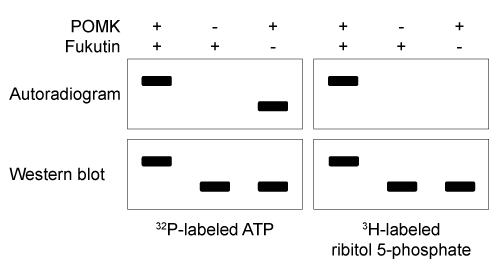 Figure 2 Autoradiograms and western blots of Dg purified from lysates incubated with 32P-labeled ATP or 3H-labeled ribitol 5-phosphate
Figure 2 Autoradiograms and western blots of Dg purified from lysates incubated with 32P-labeled ATP or 3H-labeled ribitol 5-phosphate
Adapted from Kanagawa M, Kobayashi K, Tajiri M, et al. Identification of a Post-translational Modification with Ribitol-Phosphate and Its Defect in Muscular Dystrophy. Cell Rep. 2016;14(9) :2209-23.
-POMT can use activated D-mannose, but not D-glucose, as a substrate. D-glucose and D-mannose are:
A) constitutional isomers.
B) enantiomers.
C) epimers.
D) anomers.
Correct Answer:
Verified
Q65: Passage
Upon entering muscle cells, glucose is immediately
Q66: Passage
Upon entering muscle cells, glucose is immediately
Q67: Passage
Although the structure of the cell membrane
Q68: Passage
Dystroglycan (Dg) is a transmembrane protein that
Q69: Passage
Although the structure of the cell membrane
Q71: Passage
Upon entering muscle cells, glucose is immediately
Q72: Passage
Dystroglycan (Dg) is a transmembrane protein that
Q73: Passage
Although the structure of the cell membrane
Q74: Passage
Dystroglycan (Dg) is a transmembrane protein that
Q75: Passage
Although the structure of the cell membrane
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents