Passage
The recent advent of multidrug-resistant Mycobacterium tuberculosis (Mtb) has led to the search for novel agents to combat tuberculosis. Mtb secretes over 70 serine proteases that may be used as viable drug targets. Hydrolase important for pathogenesis 1 (Hip1) is a serine protease found in Mtb with proteolytic activity that is required for dampening the host inflammatory response. Given the role of Hip1 in Mtb virulence, analyzing Hip1 substrate specificity is a critical step in designing selective inhibitors for effective tuberculosis treatment.Fluorogenic substrates are nonfluorescent compounds that release a fluorescent reporter group when acted upon by an enzyme. Such substrates are useful biomolecular imaging tools that allow monitoring of enzymatic activity in vitro and in vivo. A large library of peptides was screened against Hip1, and enzymatic cleavage was analyzed using mass spectrometry to facilitate the design of fluorogenic substrates (Figure 1) .
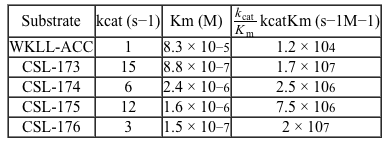
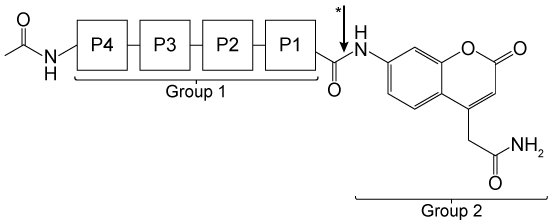 Figure 1 Schematic design of Hip1 fluorogenic substrates. P1, P2, P3, and P4 in Group 1 represent the positions of variable amino acids in the peptide. Group 2 depicts the fluorescent compound released on Hip1 cleavage.Researchers reported Michaelis-Menten kinetic parameters for substrates from two peptide families: the WKLL substrates and the CSL substrates.Table 1 Kinetic Parameters of Fluorogenic Substrates for Mtb Hip1
Figure 1 Schematic design of Hip1 fluorogenic substrates. P1, P2, P3, and P4 in Group 1 represent the positions of variable amino acids in the peptide. Group 2 depicts the fluorescent compound released on Hip1 cleavage.Researchers reported Michaelis-Menten kinetic parameters for substrates from two peptide families: the WKLL substrates and the CSL substrates.Table 1 Kinetic Parameters of Fluorogenic Substrates for Mtb Hip1
 Adapted from Lentz CS, Ordonez AA, Kasperkiewicz P, et al. Design of Selective Substrates and Activity-Based Probes for Hydrolase Important for Pathogenesis 1 (HIP1) from Mycobacterium tuberculosis. ACS Infect Dis. 2016;2(11) :807-815.
Adapted from Lentz CS, Ordonez AA, Kasperkiewicz P, et al. Design of Selective Substrates and Activity-Based Probes for Hydrolase Important for Pathogenesis 1 (HIP1) from Mycobacterium tuberculosis. ACS Infect Dis. 2016;2(11) :807-815.
-Which of the following substrates yields the most optimal catalytic turnover and catalytic efficiency, respectively?
A) CSL-173 and WKLL-ACC
B) WKLL-ACC and CSL-176
C) CSL-176 and CSL-173
D) CSL-173 and CSL-176
Correct Answer:
Verified
Q194: During anaerobic exercise, the Cori cycle connects
Q195: Passage
Leukocyte common antigen (LCA) enzymes play several
Q196: Passage
The recent advent of multidrug-resistant Mycobacterium tuberculosis
Q197: Passage
DNA polymerization is one of the most
Q198: Passage
DNA polymerization is one of the most
Q200: A single-stranded DNA oligonucleotide composed of which
Q201: Passage
The influenza A virus is an enveloped
Q202: Passage
The influenza A virus is an enveloped
Q203: Passage
Interactions between positively charged histones and negatively
Q204: Passage
Interactions between positively charged histones and negatively
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents