Passage
Interactions between positively charged histones and negatively charged nucleic acids are altered by acetylation and methylation of histone subunits. These different post-translational modifications are recognized by histone-binding proteins such as BROMO domain Adjacent to Zinc finger 2B (BAZ2B) . BAZ2B has a net negative charge but contains a lysine-rich regulatory region between its two histone-binding domains.NMR data revealed potential interactions between BAZ2B and histone subunit H3 (Figure 1) . Binding was confirmed by isothermal titration calorimetry (ITC) , which measures enthalpy of binding when a protein solution is titrated with ligand injections of equal volume and concentration. Each injection results in a negative peak as heat is released. Figure 2 shows ITC results when wild-type BAZ2B (WT) or BAZ2B with the regulatory region removed (10M+) were titrated with unmodified histone 3 (H3) or H3 that was acetylated at lysine-14 (H3K14ac) . In each case, the Hill coefficient was approximately 1.To test binding in a biological setting (in vivo) , WT and 10M+ BAZ2B were extracted from cell lysates on affinity columns containing H3 or H3K14ac covalently attached to beads. BAZ2B was then eluted and binding was quantified by Western blot (Figure 3) .
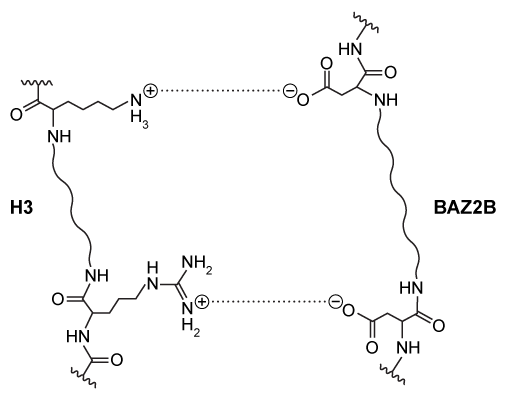 Figure 1 Electrostatic interactions between H3 and BAZ2B amino acid residues
Figure 1 Electrostatic interactions between H3 and BAZ2B amino acid residues
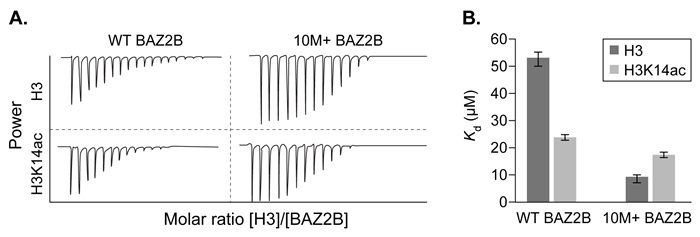 Figure 2 ITC traces (A) and Kd values (B) of WT BAZ2B and 10M+ BAZ2B titrated with either unmodified H3 or H3Ka4ac
Figure 2 ITC traces (A) and Kd values (B) of WT BAZ2B and 10M+ BAZ2B titrated with either unmodified H3 or H3Ka4ac
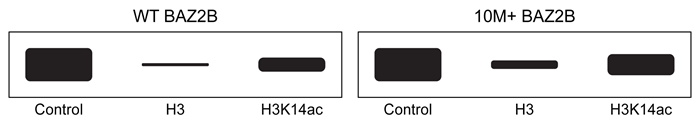 Figure 3 Western blot of BAZ2B recovered from unmodified or acetylated H3 columns
Figure 3 Western blot of BAZ2B recovered from unmodified or acetylated H3 columns
Adapted from Kostrhon S, Kontaxis G, Kaufmann T, et al. A histone-mimicking interdomain linker in a multidomain protein modulates multivalent histone binding. J Biol Chem. 2017;292(43) :17643-17657.
-Which of the proteins mentioned in the passage has the highest isoelectric point?
A) WT BAZ2B
B) 10M+ BAZ2B
C) H3
D) H3K14ac
Correct Answer:
Verified
Q201: Passage
The influenza A virus is an enveloped
Q202: Passage
The influenza A virus is an enveloped
Q203: Passage
Interactions between positively charged histones and negatively
Q204: Passage
Interactions between positively charged histones and negatively
Q205: Passage
Mitochondria are double membrane-bound cellular organelles that
Q207: Passage
Mitochondria are double membrane-bound cellular organelles that
Q208: Passage
Interactions between positively charged histones and negatively
Q209: Passage
The sirtuins are a class of enzymes
Q210: Passage
Interactions between positively charged histones and negatively
Q211: Passage
Mitochondria are double membrane-bound cellular organelles that
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents