Passage
Many cephalopods can change color to blend in with their surroundings by altering the conformations of particular proteins within their skin cells. The reflectin proteins play a major role in this camouflage mechanism. Reflectins are a group of intrinsically disordered proteins that do not adopt clearly defined secondary or tertiary structures. Under certain conditions reflectins aggregate, allowing cells to reflect various wavelengths of light.Skin cells from the squid Doryteuthis opalescens were cultured and three reflectins-DoRA, DoRB, and DoRC-were purified and analyzed to determine amino acid composition. They were found to contain an abundance of arginine and serine but relatively few cysteine, leucine, isoleucine, valine, phenylalanine, or tryptophan residues. Figure 1 shows the relative hydrophobicity of each reflectin variant as a function of amino acid position.
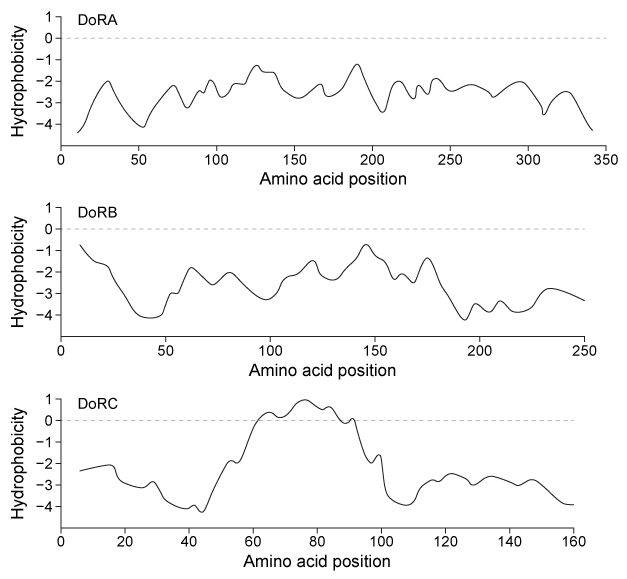 Figure 1 Hydrophobicity plots of three reflectins isolated from D. opalescens. (Note: Negative numbers indicate hydrophilic regions.) In D. opalescens skin cells (pH ≈ 7.8) , reflectins aggregate in response to acetylcholine (ACh) , which causes a signal cascade that changes the phosphorylation state of the proteins. Cells were incubated with or without ACh, and DoRA and DoRB were purified and assessed for phosphorylation by mass spectrometry. DoRA became phosphorylated in the presence of ACh whereas DoRB became dephosphorylated. After examining the isoelectric points of unmodified DoRA and DoRB, as shown in Figure 2, scientists proposed that reflectins aggregate upon charge neutralization.
Figure 1 Hydrophobicity plots of three reflectins isolated from D. opalescens. (Note: Negative numbers indicate hydrophilic regions.) In D. opalescens skin cells (pH ≈ 7.8) , reflectins aggregate in response to acetylcholine (ACh) , which causes a signal cascade that changes the phosphorylation state of the proteins. Cells were incubated with or without ACh, and DoRA and DoRB were purified and assessed for phosphorylation by mass spectrometry. DoRA became phosphorylated in the presence of ACh whereas DoRB became dephosphorylated. After examining the isoelectric points of unmodified DoRA and DoRB, as shown in Figure 2, scientists proposed that reflectins aggregate upon charge neutralization.
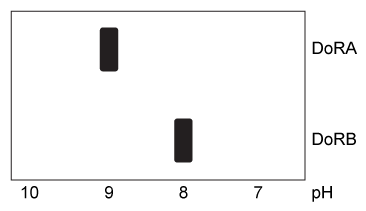 Figure 2 Isoelectric focusing of DoRA and DoRB
Figure 2 Isoelectric focusing of DoRA and DoRB
Adapted from Demartini DG, Izumi M, Weaver AT, Pandolfi E, Morse DE. Structures, Organization, and Function of Reflectin Proteins in Dynamically Tunable Reflective Cells. J Biol Chem. 2015;290(24) :15238-49.
-To generate Figure 2, reflectins were loaded onto the low-pH side of a gel with a pH gradient, and a current was applied. This resulted in:
A) gradual deprotonation as reflectins migrated from anode to cathode.
B) gradual deprotonation as reflectins migrated from cathode to anode.
C) gradual protonation as reflectins migrated from anode to cathode.
D) gradual protonation as reflectins migrated from cathode to anode.
Correct Answer:
Verified
Q226: Passage
Physical activity produces an increased energy requirement
Q227: The physical properties of two isoforms of
Q228: Passage
The ABO blood groups are determined by
Q229: An enzyme was assessed for its ability
Q230: Passage
Physical activity produces an increased energy requirement
Q232: Passage
The ABO blood groups are determined by
Q233: Passage
The ABO blood groups are determined by
Q234: Passage
The sirtuins are a class of enzymes
Q235: Passage
The sirtuins are a class of enzymes
Q236: Passage
Many cephalopods can change color to blend
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents