Passage
Destabilase, an enzyme found in the saliva of leeches, exhibits at least two activities: isopeptidase activity and glycosidase activity. Isopeptidase activity is believed to prevent blood clotting by hydrolyzing the isopeptide bond between the ε-amino group of lysine and the γ-carboxyl group of glutamate in fibrin monomers. The enzyme's glycosidase activity digests sugars found in the peptidoglycans of bacterial cell walls and may play a role in innate immunity.Two isoforms of destabilase were tagged with a polyhistidine tail and recombinantly expressed in E. coli cells. Both isoforms were then purified by affinity chromatography and eluted by imidazole, which competes with histidine-tagged proteins for column binding. Each isoform eluted efficiently with 300 mM imidazole. Five microliters of each purified enzyme were then provided with saturating levels of substrate and assessed for isopeptidase and glycosidase activity at various pH levels, which allowed the optimal pH of enzymatic activities for each isoform to be identified (Figure 1) . Further studies showed that at optimal pH, both isoforms had approximately the same kcat values, but isoform 2 had a lower Km than isoform 1 for both enzymatic activities.
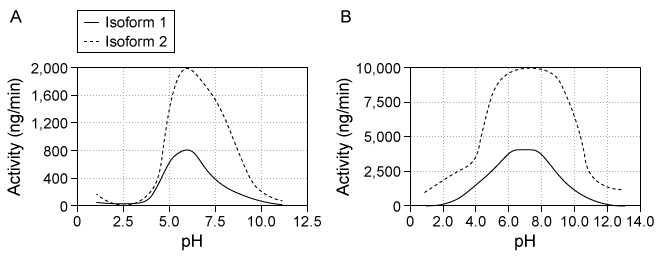 Figure 1 Isopeptidase activity (A) and glycosidase activity (B) of 5 μL of destabilase isoforms 1 and 2 at various pH valuesIsoform 2 was further characterized by mutating specific residues and measuring enzymatic activities at optimal pH. The results are shown in Figure 2.
Figure 1 Isopeptidase activity (A) and glycosidase activity (B) of 5 μL of destabilase isoforms 1 and 2 at various pH valuesIsoform 2 was further characterized by mutating specific residues and measuring enzymatic activities at optimal pH. The results are shown in Figure 2.
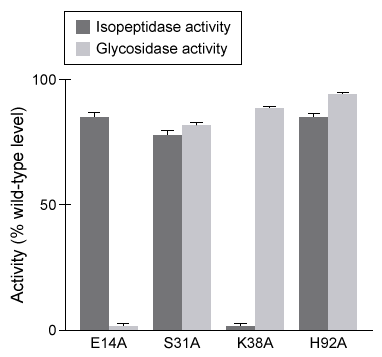 Figure 2 Isopeptidase and glycosidase activity of isoform 2 of destabilase with various mutations
Figure 2 Isopeptidase and glycosidase activity of isoform 2 of destabilase with various mutations
-Both isoforms of destabilase have approximately the same kcat values for isopeptidase activity at optimal pH, but Figure 1 shows that when 5 μL of each purified enzyme was provided with saturating levels of substrate, the isoforms had different levels of activity. What factor could explain this apparent discrepancy?
A) Isoform 2 had less activity than isoform 1 because isoform 2 did not operate at Vmax under saturating conditions.
B) The 5 μL sample of isoform 1 had a higher enzyme concentration than the isoform 2 sample because the affinity column bound isoform 1 more tightly.
C) Isoform 1 had less activity than isoform 2 because isoform 1 became denatured at pH 6.
D) The 5 μL sample of isoform 2 had a higher enzyme concentration than the isoform 1 sample because E. coli expressed isoform 2 at higher levels.
Correct Answer:
Verified
Q295: Passage
Destabilase, an enzyme found in the saliva
Q296: Passage
Glycolysis and gluconeogenesis are tightly regulated, opposing
Q297: Certain RNA molecules can fold into structures
Q298: An enzyme generates a Lineweaver-Burk plot with
Q299: Passage
Glycolysis and gluconeogenesis are tightly regulated, opposing
Q301: Passage
The HIV-derived transactivator of transcription (Tat) is
Q302: Passage
The HIV-derived transactivator of transcription (Tat) is
Q303: The electron transport chain results in the
Q304: Passage
The HIV-derived transactivator of transcription (Tat) is
Q305: Passage
The HIV-derived transactivator of transcription (Tat) is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents