Passage
The bicarbonate (HCO3−) buffer system (Reaction 1) helps to maintain acid-base homeostasis and a blood pH near 7.4. Therefore, concentrations of carbon dioxide (CO2) and HCO3− must be tightly regulated through adaptations in respiratory and renal physiology. For patients with suspected acid-base imbalance, these concentrations can be monitored by blood gas analysis performed on arterial or venous blood.
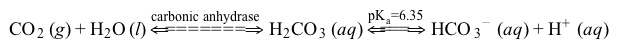 CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1) . The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
CO2g+H2Ol⇔carbonic anhydraseH2CO3aq⇔pKa=6.35HCO3-aq+H+aqReaction 1In most automated blood gas analyzers, ionized hydrogen, CO2, and oxygen (O2) in the sample diffuse through semipermeable membranes and are measured at separate electrodes (Figure 1) . The pH-sensitive glass electrode (E1) is separated from the blood sample by a membrane permeable to hydrogen ions. The sample pH is determined according to the voltage difference between E1 and a reference electrode maintained in a solution of standard pH. CO2 diffuses across a gas-permeable membrane to E2, where it reacts to generate HCO3− and free hydrogen ions. In a modification of the mechanism used in E1, partial pressure of CO2 (PaCO2) is calculated indirectly from the change in pH, as determined by the potential difference between E2 and its reference electrode.E3, also called the Clark electrode, is an electrochemical cell containing a silver anode and a platinum cathode. O2 diffuses through another gas-permeable membrane and reacts with hydrogen ions to form water. The current measured at E3 is then used to calculate PaO2 in the blood sample.
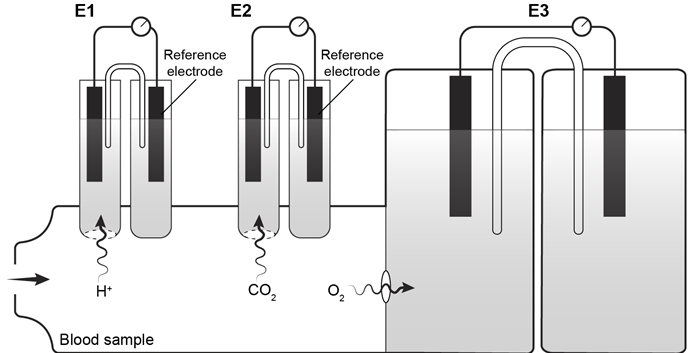 Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3) , reported in milliequivalents per liter (mEq/L) , can be calculated from PaCO2 by Equation 1:
Figure 1 Automated blood gas analyzerThe concentration of carbonic acid (H2CO3) , reported in milliequivalents per liter (mEq/L) , can be calculated from PaCO2 by Equation 1:
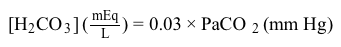 H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
H2CO3mEqL=0.03×PaCO2mm HgEquation 1Subsequently, using the concentration of H2CO3 obtained from Equation 1, the HCO3− concentration resulting from decomposition of carbonic acid can then be calculated by applying the Henderson-Hasselbalch equation.
-If a 0.2-mL blood sample with a PaCO2 of 40 mm Hg diffuses across a membrane into the E2 electrode containing 0.3 mL of solution, the final PaCO2 in the blood sample will be:
A) exactly 0 mm Hg.
B) less than 16 mm Hg.
C) exactly 16 mm Hg.
D) greater than 16 mm Hg.
Correct Answer:
Verified
Q4: Passage
Depending on the active compound's specific method
Q5: Passage
Nuclear medicine uses radiopharmaceuticals for disease treatment
Q6: Passage
The bicarbonate (HCO3−) buffer system (Reaction 1)
Q7: Passage
Magnetic resonance imaging (MRI) interprets the nuclear
Q8: Passage
Magnetic resonance imaging (MRI) interprets the nuclear
Q10: Passage
Magnetic resonance imaging (MRI) interprets the nuclear
Q11: Passage
The bicarbonate (HCO3−) buffer system (Reaction 1)
Q12: Passage
Magnetic resonance imaging (MRI) interprets the nuclear
Q13: Passage
The bicarbonate (HCO3−) buffer system (Reaction 1)
Q14: Passage
Depending on the active compound's specific method
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents