Passage Combustion Occurs When an Oxidation-Reduction Reaction Takes Place Between a a Reduced
Passage
Combustion occurs when an oxidation-reduction reaction takes place between a reduced fuel source and an oxidizer, most frequently oxygen. The reaction between hydrogen and oxygen forms water (Reaction 1) and is highly exothermic and thermodynamically favorable. At the completion of hydrogen combustion, essentially all H2 molecules have been converted to water. Nevertheless, at room temperature and atmospheric pressure, molecular hydrogen and oxygen can coexist quite stably.2 H2 + O2 → 2 H2OReaction 1A group of researchers designed two prototype internal combustion engines, each of which could inject a hydrogen/oxygen mixture into a 5-mL combustion chamber at a total pressure of 1 atm and could repeat this process for several cycles. In one engine design, a spark was generated to initiate combustion as hydrogen and oxygen were injected. In another method, the combustion chamber was coated with a small amount of platinum powder. Platinum dramatically increases the reaction rate at room temperature without the need for a spark. The platinum itself is not altered by the reaction and can be reused. Researchers measured the maximum power output of both engine designs under identical injection and temperature conditions (Figure 1) .
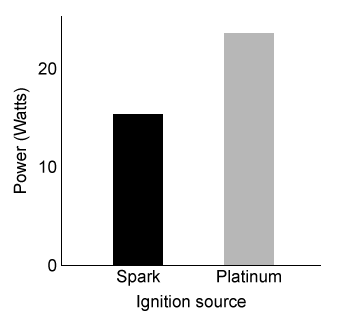 Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
Figure 1 Relative power outputs of hydrogen engines with different ignition sources at 298 KThe power output of an engine is directly related to the rate of combustion. In the presence of platinum, researchers measured the engine's rate of water production at various temperatures and H2/O2 mixture compositions. The results are shown in Figure 2.
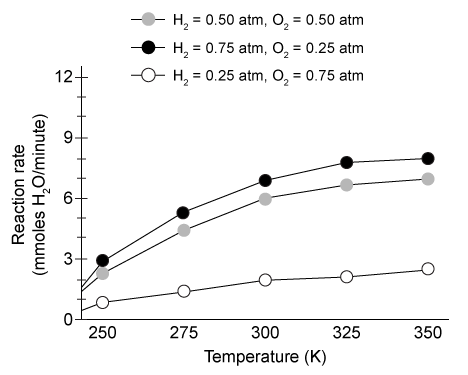 Figure 2 Rate of combustion in platinum-coated engines under various conditions
Figure 2 Rate of combustion in platinum-coated engines under various conditions
Adapted from Schultze M, Mantzaras J. Hetero-/homogeneous combustion of hydrogen/air mixtures over platinum: Fuel-lean versus fuel-rich combustion modes doi: 10.1016/j.ijhydene.2013.06.069
-In the absence of platinum, a spark initiates combustion by doing which of the following?
A) It shifts the reaction equilibrium toward water formation.
B) It consumes oxygen, driving the reaction toward the products.
C) It increases the average kinetic energy of nearby molecules.
D) It removes electrons from oxygen to form reactive oxygen species.
Correct Answer:
Verified
Q72: Passage
Kidney stones are a common ailment affecting
Q73: Passage
Household cleaners commonly contain either ammonia (NH3)
Q74: Passage
Combustion occurs when an oxidation-reduction reaction takes
Q75: Passage
Household cleaners commonly contain either ammonia (NH3)
Q76: Passage
Kidney stones are a common ailment affecting
Q78: Passage
Combustion occurs when an oxidation-reduction reaction takes
Q79: Passage
Combustion occurs when an oxidation-reduction reaction takes
Q80: Passage
An automated external defibrillator (AED) is a
Q81: A radioactive atom decays by 5 alpha,
Q82: The bonds of four salts (MgBr2, NaCl,
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents