Passage
An automated external defibrillator (AED) is a medical device used to send an electric shock to the heart after cardiac arrest. A key component of the AED is the power source, or battery. Batteries used in AEDs need to have a good charge-to-weight ratio; they must be safe and reliable as well as rechargeable.Two types of rechargeable batteries used in early models of AEDs are the lead storage (also called lead-acid) battery and the nickel-cadmium (NiCd) battery. These batteries consist of multiple electrochemical cells that are connected in series to deliver a potential between 9 V and 18 V. A capacitor allows the AED to accumulate charge so that it can deliver between 300 V and 1,000 V.Some AEDs use a sealed lead storage battery. Lead storage batteries are robust and hold a charge for a long time. However, they have a low energy-to-weight ratio. Each lead storage cell delivers approximately 2.0 V, and a battery of four cells weighing 1000 g can provide 30 W∙h of energy.
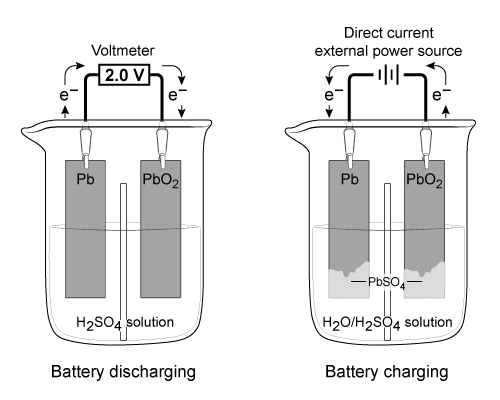 Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
Figure 1 Electron flow in a lead storage battery when discharging and chargingThe half reactions for the anode and cathode of a lead storage battery in 4 M of sulfuric acid (H2SO4) are shown in Reactions 1 and 2:
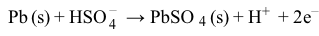 Reaction 1
Reaction 1
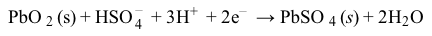 Reaction 2
Reaction 2
NiCd batteries have a higher energy-to-weight ratio than lead storage batteries but cannot hold as much charge. Each NiCd cell delivers approximately 1.3 V, and a single-cell battery weighing 120 g can provide 7.2 W∙h of energy.The half reactions at the anode and cathode for a NiCd battery in KOH are shown in Reactions 3 and 4:
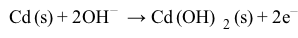 Reaction 3
Reaction 3
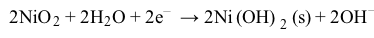 Reaction 4
Reaction 4
-If the electromotive force of the battery in an AED is found to be −2.0 V while it is charging, the battery is functioning as a:
A) galvanic cell.
B) electrolytic cell.
C) concentration cell.
D) fuel cell.
Correct Answer:
Verified
Q66: Passage
An automated external defibrillator (AED) is a
Q67: Passage
An automated external defibrillator (AED) is a
Q68: Passage
Combustion occurs when an oxidation-reduction reaction takes
Q69: Passage
Kidney stones are a common ailment affecting
Q70: Passage
Household cleaners commonly contain either ammonia (NH3)
Q72: Passage
Kidney stones are a common ailment affecting
Q73: Passage
Household cleaners commonly contain either ammonia (NH3)
Q74: Passage
Combustion occurs when an oxidation-reduction reaction takes
Q75: Passage
Household cleaners commonly contain either ammonia (NH3)
Q76: Passage
Kidney stones are a common ailment affecting
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents