Consider the dimerization of nitrogen dioxide at room temperature: 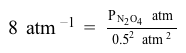 2 NO2g⇌ N2O4g Keq = 8 atm-1If the equilibrium partial pressure of nitrogen dioxide in a container is 0.5 atm, what is the partial pressure of dinitrogen tetroxide?
2 NO2g⇌ N2O4g Keq = 8 atm-1If the equilibrium partial pressure of nitrogen dioxide in a container is 0.5 atm, what is the partial pressure of dinitrogen tetroxide?
A) 0.03 atm
B) 0.5 atm
C) 2 atm
D) 4 atm
Correct Answer:
Verified
Q94: Passage
Kidney stones are a common ailment affecting
Q95: Passage
Dmitri Mendeleev is credited with grouping the
Q96: A combination reaction was performed as described
Q97: The regulation of mineral ions in the
Q98: Passage
Dmitri Mendeleev is credited with grouping the
Q100: Passage
Kidney stones are a common ailment affecting
Q101: Passage
A group of students were given water
Q102: Passage
Cyanide (CN−) is a powerful, rapid toxin
Q103: Passage
Although acetylsalicylic acid (aspirin) is a well-known
Q104: Passage
Cyanide (CN−) is a powerful, rapid toxin
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents