Passage
Dmitri Mendeleev is credited with grouping the elements by atomic mass and observing that similar properties emerge periodically. Henry Moseley then determined that a fundamental property, which Ernest Rutherford later called the atomic number, is more important than atomic mass for ordering the elements.As the periodic table took shape, scientists noted certain properties and trends. For example, Rayleigh and Ramsay observed that some elements are unreactive and gave them the name noble gases. But it was not until Niels Bohr described his model of the atom that scientists understood why noble gases are stable.Another important trend was discovered by Linus Pauling. He was the first to identify electronegativity as a way to describe bonds that are neither completely ionic nor completely covalent. Pauling assigned fluorine the highest electronegativity value of 4.0 because of its strong tendency to attract electrons when bonded to other atoms. All other atoms were assigned values relative to fluorine. This became known as the Pauling electronegativity scale.Scientists identified other trends based on x-ray crystallography and reactivity studies. Figure 1 shows the first ionization energy of the elements, and Figure 2 shows electron affinity.
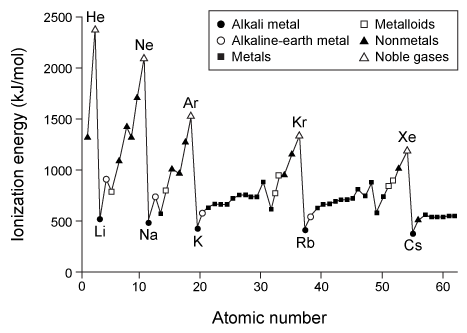 Figure 1 First ionization energy of elements
Figure 1 First ionization energy of elements
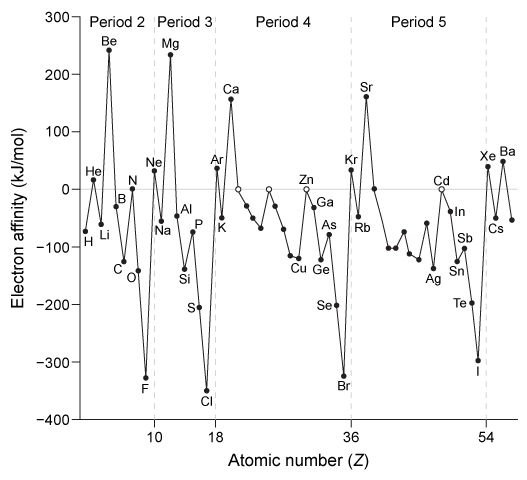 Figure 2 Electron affinity of several elements (larger negative values indicate greater electron affinity)
Figure 2 Electron affinity of several elements (larger negative values indicate greater electron affinity)
-Why are noble gases the least reactive group on the periodic table?
A) Noble gases are diamagnetic.
B) Noble gases have a full valence shell.
C) Noble gases tend to have the same number of protons and neutrons.
D) Noble gases tend to form diatomic molecules.
Correct Answer:
Verified
Q90: A reaction was performed in which compounds
Q91: An 84-mg sample of a compound is
Q92: Antacids act by functioning chemically as weak
Q93: Passage
Dmitri Mendeleev is credited with grouping the
Q94: Passage
Kidney stones are a common ailment affecting
Q96: A combination reaction was performed as described
Q97: The regulation of mineral ions in the
Q98: Passage
Dmitri Mendeleev is credited with grouping the
Q99: Consider the dimerization of nitrogen dioxide at
Q100: Passage
Kidney stones are a common ailment affecting
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents