Copper plays a vital role as a trace mineral for biological processes, including the electron transport chain and collagen synthesis. The Pourbaix diagram below outlines the relationship between several copper species as a function of solution pH and applied potential. Which statement accurately describes the chemical changes shown in this diagram? 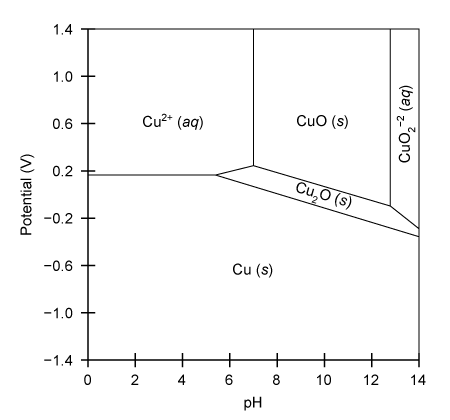
A) The predominant species at a potential of −0.2 V and pH of 13, is CuO.
B) Cu2+ is reduced and precipitates as Cu2O as pH increases to a pH of 8 at a potential of +0.3 V.
C) Cu is reduced to Cu2+ as the potential is changed from −0.4 to +0.2 at a pH of 4.
D) The equilibrium between Cu2+ and CuO is independent of applied potential above +0.3 V at a pH of 7.
Correct Answer:
Verified
Q127: Passage
Gastric acid is one of many acids
Q128: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q129: Which compound has the bond with the
Q130: A Galileo thermometer consists of a cylinder
Q131: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q133: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q134: Passage
Gastric acid is one of many acids
Q135: Which of the following element groups is
Q136: Passage
Gastric acid is one of many acids
Q137: Which of the following statement(s) is (are)
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents