A Galileo thermometer consists of a cylinder filled with a known solution and several sealed glass bulbs partially filled with water. Each bulb effectively acts as an isolated system. If one of the bulbs has the same density as the solution at 25 °C, how will its position and the density of the solution change as the temperature of the solution decreases from 25 °C to 20 °C? 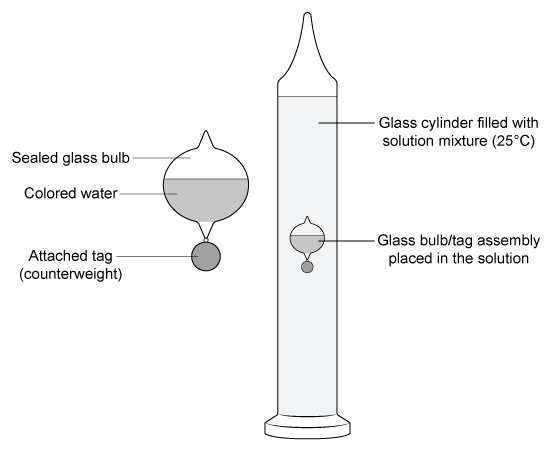
A) The cylinder solution density will decrease, and the glass bulb will sink.
B) The cylinder solution density will decrease, and the glass bulb will float.
C) The cylinder solution density will increase, and the glass bulb will sink.
D) The cylinder solution density will increase, and the glass bulb will float.
Correct Answer:
Verified
Q125: Passage
Gastric acid is one of many acids
Q126: Passage
Gastric acid is one of many acids
Q127: Passage
Gastric acid is one of many acids
Q128: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q129: Which compound has the bond with the
Q131: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q132: Copper plays a vital role as a
Q133: Passage
Potassium permanganate (KMnO4) is a highly reactive
Q134: Passage
Gastric acid is one of many acids
Q135: Which of the following element groups is
Unlock this Answer For Free Now!
View this answer and more for free by performing one of the following actions

Scan the QR code to install the App and get 2 free unlocks

Unlock quizzes for free by uploading documents